"Induction by fibroblast growth factors of mesoderm during gastrulation leads to blood-forming tissue, including angioblasts & hemopoietic cells, that together constitute the blood islands of the yolk sac." (Vasculogenesis, Werner Risau & Ingo Flamme, Annual Review of Cell Division Biology, 1995, p. 73)
The yolk sac actually reproduces the essential first blood cells for the new individual--an activity that is functionally quite different from the bird egg yolk.
For example, careful experiments have shown that the epidermis ANYWHERE on the embryo can form a limb if the appropriate developmental signal is given. The fact the (same) signal is given in different segments does not mean that vertebrate limbs are not homologous. The limbs themselves develop from the exact same tissues in each of the embryos you cited, and develop into homologous bone and tissue patterns. Ken Miller responding to my homology argument of limbs growing from different sections.
|
|
|
|

|
Proof that textbooks in use this school year engage in classroom fraud. |
|
|
|
|
Vertebrates, Kenneth V. Kardong, 1998, ISBN 0-697-28654-1, McGraw-Hill
In use April 2000 2nd - 4th year biology at McMaster University. |
EXTRAEMBRYONIC MEMBRANES: Mammals. Structures homologous to the four extraernbryonic membranes of reptiles and birds appear in mammals: amnion, chorion, yolk sac, and allantois. In placental mammals, the amnion forms over the embryo. In some placental mammals, Such as dogs and pigs, it forms from amniotic folds in the somatopleure after the embryo is well organized. In other placental mammals, such as humans, fluid, filled spaces appear within the inner cell mass prior to the establishment of germ layers. These spaces coalesce to form the initial amniotic cavity. In monotremes, the extraembryonic membranes are formed in much the same way they are formed in reptiles and birds (table 5-4). In therian mammals, marsupials and placentals, a structure homologous to the yolk sac is present, but it contains no yolk platelets. Instead, it is filled with fluid. The embryonic disc is suspended between the amniotic cavity and the yolk sac. As in other amniotes, the atlantois begins as an outgrowth of the hindgut that expands outward, becoming surrounded by a layer of mesoderm as it grows. The chorion of placental mammals is bilaminar, as in reptiles and birds, but it forms from the trophoblast and includes the adjacent mesodermal layer. The expanding allantois grows into contact and fuses with much of the internal wall of the chorion, producing the chorioallantoic membrane. The allantoic vessels, or the umbilical vessels, as they are more often called in mammals, develop within the mesodermal core of the chorioallantoic membrane. These vessels function in respiration and nutritional exchange with the uterus of the mother. (Vertebrates, Kenneth V. Kardong, 1998, ISBN 0-697-28654-1, McGraw-Hill, p 183)

(click on photo for high resolution)
Most people are surprised to learn that placentae develop in marsupials and even in some fishes, amphibians, and reptiles . In fact, birds are the only vertebrate class in which no members possess a placenta. (Vertebrates, Kenneth V. Kardong, 1998, ISBN 0-697-28654-1, McGraw-Hill, p 184)
OVERVIEW OF EARLY EMBRYONIC DEVELOPMENT: In eutherian mammals, the yolk sac is almost entirely devoid of yolk, yet cleavage is discoidal and gastrulation occurs via a primitive streak just as if large quantities of yolk were present and cells had to move around such an obstruction. This cleavage process likely represents the retention of features inherited from ancestors with yolk-laden eggs. Without reference to the phylogenetic background of eutherian mammals, such a pattern of early embryonic development would be difficult to explain. (Vertebrates, Kenneth V. Kardong, 1998, ISBN 0-697-28654-1, McGraw-Hill, p 185)

(Click on photo for high resolution)
"FIGURE 5.38 Haeckel's comparison of early embryonic stages across vertebrate groups. Eight species are shown across the figure. The youngest developmental stage of each is at the top of the figure followed by two successively older stages below. After Haekel." (Vertebrates, Kenneth V. Kardong, 1998, ISBN 0-697-28654-1, McGraw-Hill, p 191)
ONTOGENY AND PHYLOGENY: Biogenetic Law It has long been supposed that ontogeny, especially early events of embryonic development, retains current clues to distant evolutionary events. Ernst Haeckel, a nineteenth-century German biologist, stated this boldly in what became known as the biogenetic law. Pharyngeal slits, numerous branchial arches, and other fish characteristics even appear in the early embryos of reptiles, birds, and mammals, but they are lost as these tetrapod embryos proceed to term (figure 5.38). Although lost as tetrapod development unfolds, these and many similar structures are remnants of fish features from the evolutionary past. Haeckel argued that from ovum to complete body, the individual passes through a series of developmental stages that are brief, condensed repetitions of stages through Which its successive ancestors evolved. The biogenetic law states that ontogeny in abbreviated form recapitulates (repeats) phylogeny. Haeckel certainly recognized that recapitulation was approximate. Comparing it to an alphabet, lie suggested that the ancestry behind each organism might be a sequence of stages: A, B, C, D, E.... Z, whereas the embryology of a descendant individual might pass through an apparently defective series: A, B, D, F, H, K, M etc. In this example, several evolutionary stages have fallen Out of the develop-mental series. Although the ancestry of an organism might include an entire series of steps, Haeckel did not believe that all these would necessarily appear in the ontogeny of a later individual. Evolutionary stages Could disappear from the developmental series. Nevertheless, he felt that the basic series of major ancestral stages remained the same, and thus the biogenetic law applied. Development certainly exhibits a conservatism wherein ancient features persist like heirlooms in modern groups. Ontogeny, however, is not so literally a repeat of phylogeny as Haeckel supposed. A contemporary of his, Karl Ernst von Baer, cited examples from embryos of descendant animals that did not conform to the biogenetic law: chick embryos lack the scales, swim bladders, fin rays, and so forth of adult fishes that evolutionarily preceded them. Furthermore, the order of appearance of ancestral structures is sometimes altered in descendant embryos. Haeckel al-lowed for exceptions; von Baer did not. Von Baer said that these exceptions and "thousands" more were too much. He proposed alternative laws of development. Foremost was von Baer's proposal that development proceeds from the general to the specific. Development begins with undifferentiated cells of the blastula that become germ layers, then tissues, and finally organs. Young embryos are undifferentiated (general), but as development proceeds, distinguishing features (specific) of the species appear. Each embryo, in-stead of passing through stages of distant ancestors, departs more and more from them. Thus, the embryo of a descendant is never like the adult of an ancestor and only generally like the ancestral embryo. Other scientists since von Baer have also dissented from strict application of the biogenetic law. What can be made of all this? We should recognize that embryos are adapted to their environments. Anamniotes usually make their homes in salt, or fresh water. Amniote embryos grow in an aquatic environment as well, the amniotic fluid. Thus, similarities between embryos might be expected. But, as von Baer pointed out, what we observe at best is correspondence between the embryos of descendants and the embryos of their ancestors, not between the descendant embryos and the ancestral adults. Haeckel was certainly mistaken to see a correspondence between descendant embryos and ancestor adults. Organs are adapted to their functions. As a fish embryo approaches hatching, its "limb" buds become fins, a bird's become wings, a mammal's become paws or hooves or hands, and so forth. There is, however, an element of conservatism in ontogeny, even if it is not an exact telescoping of evolutionary events. After all, the young embryos of mammals, birds, and reptiles do develop pharyngeal slits that never become functional as respiratory devices. Is this recapitulation? No. It is better to think of this as preservationism for reasons not too difficult to imagine. Each adult part is the developmental product of prior embryonic preparation. The zygote divides to form the blastula; gastrulation brings germ layers to their proper positions; mesoderm interacts with endoderm to form organ rudiments; tissues within organ rudiments differentiate into adult organs. Skip a step, and the whole cascade of ensuing developmental events may fail to unfold properly. In mammals, the notochord of the embryo is replaced almost entirely in the adult by the solid vertebral Column (figure 5.39). For the young embryo, the notochord provides an initial axis, a scaffolding along which the delicate body of the embryo is laid Out. The notochord also stimulates (development of the overlying nerve tube. If the notochord is re-moved, the nervous system does not develop. The adult supportive role is taken over by the vertebral Column, but the notochord performs a vital embryonic role before disappear-ing; namely, it serves the Young embryo as a central element of embryonic organization. A notochord that persists in the mammalian embryo should not be interpreted as a sentimental memento of a distant phylogenetic history. Instead, it should be seen as a functioning component of early embryonic development. Structures and processes intertwine to produce the conservatism evident in development. They are not easily eliminated without a broad disruption of ensuing events. Anatomical innovations, new structures brought into service in the adult, are usually added at the end of developmental processes, not at the beginning. A new structure inserted early into the developmental process would require many simultaneous replacements of many disrupted developmental processes thereafter. Evolutionary innovations thus usually arise by remodeling rather than by entirely new construction. The forelimbs of ancestors that supported the body and allowed the organism to romp over the surface of the land are renovated into the wings that carry bats and birds aloft. We need look no further than our own human bodies to find similar examples of evolutionary remodeling. The backbone and legs that carried our distant ancestors comfortably on all fours hold us upright in a bipedal stance. The arms and hands that can control the delicate strokes of a paintbrush or the writing of a novelist come refashioned from ancient forelegs that carried a hefty trunk and helped our ancestors dash from predators. The past is hard to erase. When parts are already available, renovation is easier than new construction. (Vertebrates, Kenneth V. Kardong, 1998, ISBN 0-697-28654-1, McGraw-Hill, p 190-192) |
|
|
|
|
Evolutionary Biology, Douglas J. Futuyma, Third Edition, 1998 ISBN: 0-87893-189-9
In use April 2000 2nd - 4th year biology at McMaster University. |
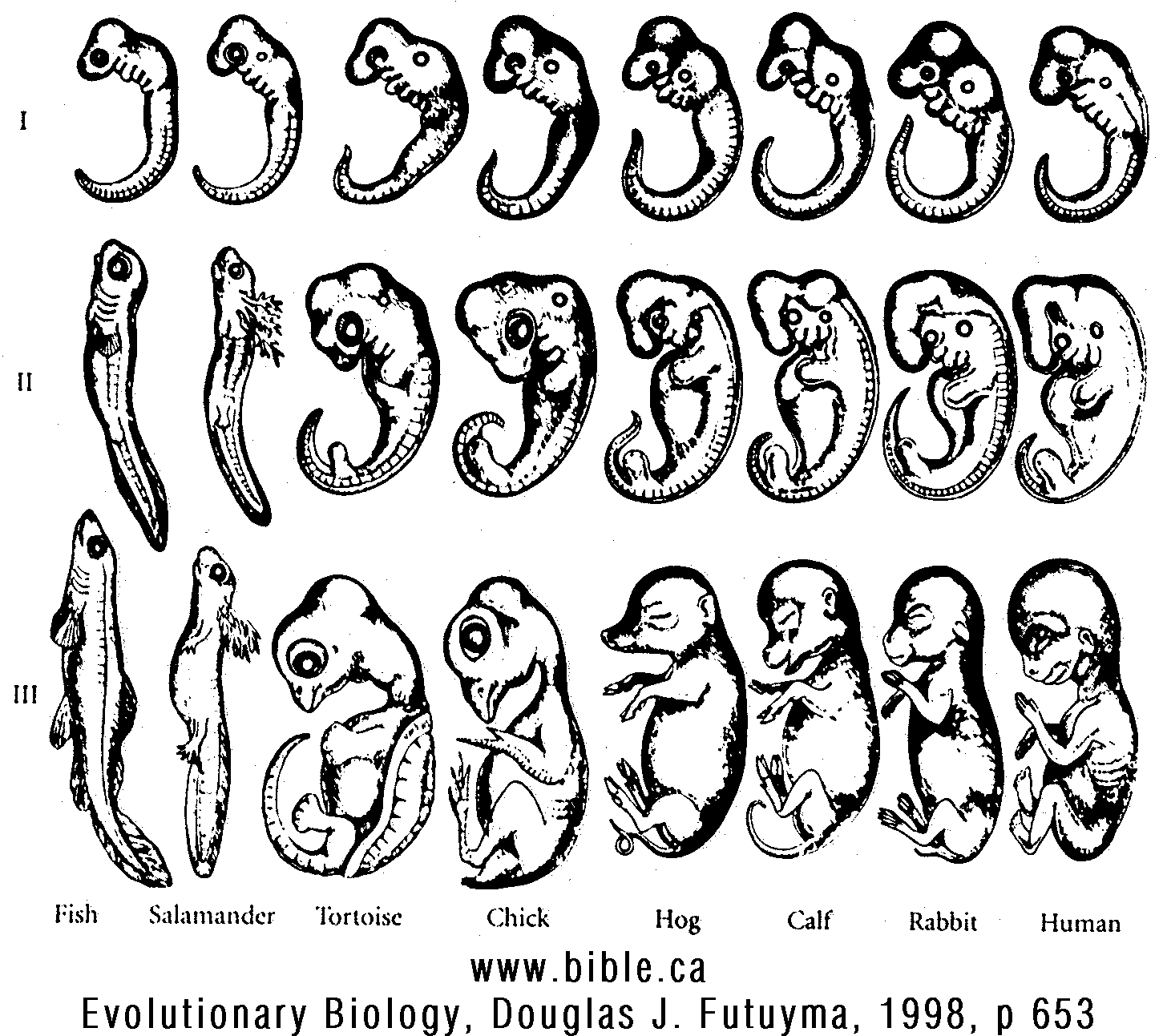
"FIGURE 23.1 (Fish, Salamander, Tortoise, Chick, Hog, Calf, Rabbit, Human): An illustration of von Baer's law: three stages in the development of several vertebrates. All the vertebrate classes share many common features early in development; many distinguishing features of the classes and orders appear later. (From Romanes 1901.) (Evolutionary Biology, Douglas J. Futuyma, Third Edition, 1998 ISBN: 0-87893-189-9, p 653)"
"Several kinds of information are necessary to account for both the similarities and the differences among organisms. ... Of all the major fields of biology that bear on evolutionary theory, developmental biology has been least incorporated into evolutionary science until very recently. Darwin declared in a letter to the botanist Asa Gray that "embryology is to me by far the strongest single class of facts in favor of a change of forms," and his followers, especially Ernst Haeckel, used embryological similarities as a major source of evidence for phylogenetic relationships (see below). However, when, early in the twentieth century, embryology made the transition from a purely descriptive field to an experimental science concerned with discovering the mechanisms of development, developmental and evolutionary studies diverged. During the Evolutionary Synthesis, evolutionary biologists focused on population genetics, and may not have known how to assimilate developmental data into their evolutionary theory. With a few exceptions-notably C. H. Waddington (1957)--experimental embryologists made few attempts to relate their data to evolutionary theory. Worse yet, the perspectives of embryologists, with their focus on mechanisms that operate within individual organisms, and of evolutionary theorists, who stress gradual gene frequency change at the population level, have often seemed incompatible. This tension still pervades some recent discussions of the role of development in evolution (cf Goodwin et al. 1983; Ho and Saunders 1984). Only in about the last decade has developmental biology begun again to play a major role in evolutionary biology. The emerging field of "evolutionary embryology" (MuIler 1991), or "evolutionary developmental biology" (Raff 1996), seeks to determine how embryological processes have been modified in evolution and how develop-mental mechanisms have influenced the course of evolution. Developmental explanations are complementary, not mutually exclusive, to explanations in terms of molecular and population genetics. For example, an experimental embryologist may show that the form of a bone depends on the stresses that muscles impose on it during development. It would be wrong, though, to conclude that differences among species in the shape of this bone lack a genetic basis. Allele substitutions can alter the bone's shape via their effect on the intermediate processes (such as muscular insertions and stresses) that give it form. Furthermore, although developmental and genetic descriptions account for the PROXIMATE mechanisms that determine bone shape, they do not explain why alleles effecting one bone shape rather than another characterize the species. For this, we require an ULTIMATE explanation, such as natural selection or genetic drift. APPROACHES TO STUDYING DEVELOPMENT AND EVOLUTION On the basis of comparisons of the morphological features of different organisms, evolutionary biologists since the late nineteenth century have developed descriptive models of changes in development whereby an ancestral morphology might be transformed into a derived morphology. For instance, the greatly elongated digits that support the membrane of a bat's wing suggest a model in which the growth rate of the digits, relative to that of the rest of the body, increased during the origin of bats. Such formal models are not based on information about the mechanisms of development, but they may suggest mechanisms, such as elevated rates of cell division in the developing fingers of bats. Formal models of this kind still play an important role in describing patterns of evolutionary change in morphology. Beginning early in the twentieth century, developmental biologists used the methods of experimental embryology to gain insights into the mechanisms of development, through manipulations such as removing (ablating) parts of embryos or transplanting organ rudiments between embryos. By such means, it was discovered, for example, that the developing dermis of vertebrate embryos, derived from mesoderm, induces the epidermis, which is derived from ectoderm, to develop scales, hairs, or feathers. In a few in-stances, a chemical basis for such processes was deter-mined; for example, it was found that thyroxin, secreted by the thyroid gland, triggers metamorphosis of amphibian larvae to the adult form. Provided with such information, evolutionary biologists could sometimes gain insight into evolutionary changes in developmental pathways. For example, some salamander species achieve reproductive maturity without undergoing metamorphic change in the rest of their morphology. As predicted, such salamanders pro-duce little or no thyroxin. Insights from the methods of classic experimental embryology, however, usually do not provide descriptions of developmental processes at the molecular level. In the past decade, some of the most exciting contemporary advances in all of biology have been made by using the techniques of molecular biology to reveal how genes affect development. Evolutionary biology has both contributed to these advances and profited from them, for we are beginning to learn how developmental processes evolve at a genetic level. We shall describe the developmental bases of evolutionary change first in macroscopic, nonmolecular terms, and then in terms of contemporary molecular genetics. ONTOGENY AND PHYLOGENY Before The Origin of Species, biologists were already well aware that species are often more similar as embryos than as adults. Karl Ernst von Baer noted in 1828 that the features common to a more inclusive taxon (such as the subphylum Vertebrata) often appear in development before the specific characters of lower-level taxa (such as orders or families). This generalization is now known as VON BAER'S LAW. Probably the most widely known example is the similarity of many tetrapod vertebrate embryos, which display gill slits, a notochord, segmentation, and paddle-like limb buds be-fore the features typical of their class or order become apparent (Figure 23.1). Another example is provided by the metatarsals of both birds and artiodactyls (e.g., cows), which are initiated as separate elements like those of other tetrapods, and only later become fused into the single bone characteristic of these taxa. Darwin viewed embryological similarities as the most important source of evidence for the reality of evolution, and cited many examples in The Origin of Species. The relation between embryology and evolution was developed at length by one of Darwin's most enthusiastic supporters, the German biologist Ernst Haeckel. Haeckel coined the words ontogeny (the development of the individual organ-ism) and phylogeny (the evolutionary history of species), and in 1866 issued his famous BIOGENETIC LAW: "Ontogeny re-capitulates phylogeny." By this, Haeckel meant that in the course of its development, an individual successively passes through the adult forms of all its ancestors, from the very origin of the first cell to the present. Haeckel thus sup-posed that by studying embryology, one could read a species' phylogenetic history, and therefore infer directly phylogenetic relationships among organisms. Repeated in biology textbooks ever since, Haeckel's "law" is one of the most famous maxims in biology. But by the end of the nineteenth century, it was already clear that the law rather seldom holds. The real development of organisms differs in several important ways from Haeckel's simple scheme (Gould 1977): 1. Adult features of ancestors seldom appear as inter-mediate stages in the ontogeny of a descendant. von Baer's law is more often closer to the truth: different descendants of a common ancestor are likely to share early embryonic characters with one another and, presumably, with their ancestor. For example, although the embryos of mammals, "reptiles," and fishes all have gill pouches, in mammals and "reptiles" neither these features nor any others even fleetingly acquire the form typical of adult fishes. 2. Various features develop at different rates, relative to each other, in descendants than in their ancestors. Thus the ancestral morphology as a whole does not make a coordinated appearance during ontogeny. We shall shortly treat this subject under the topic of heterochrony (another word that Haeckel coined). 3. The pre-adult stages of any organism must grow and survive; to do so, they require stage-specific adaptations suitable for the particular environment of the species. Thus the horny beak of tadpoles, the innumerable adaptations of the larvae of insects and other invertebrates, and the deciduous egg tooth used by birds to break out of the eggshell are newly evolved features in these several lineages. In the extreme case, intermediate stages in the ontogeny of an ancestor may be lost completely from the ontogeny of a descendant; for example, direct-developing sea urchins such as Heliocidaris erythrogramma arrive at the adult morphology without passing through the planktonic larval stage that is typical of the ancestral ontogeny. 4. Haeckel's biogenetic law postulates that phylogenetically new features are added to the end of the ancestral ontogeny (TERMINAL ADDITION). The strongest blow to Haeckel's law was dealt by the discovery of paedomorphic species (from paedos, "child") -species in which the juvenile morphology of the ancestor is retained throughout life. In these cases, there has been terminal subtraction of stages of the ancestral ontogeny. The best-known examples are axolotls, members of the salamander genus Ambystoma in which metamorphosis does not occur, and larval features such as gills and the tail fin are retained throughout reproductive life (Figure 23.2). In the ancestral ontogeny, displayed by most species of Ambystoma, the aquatic larva metamorphoses into a terrestrial adult that lacks gills and the tail fin. There are, to be sure, many cases in which certain features of an ancestor are recapitulated in the ontogeny of a descendent; for example, the metatarsals of a bird, as we saw above, at first develop separately (the ancestral condition) be-fore becoming fused together. Still, the biogenetic law is honored more often in the breach than in the observance, and it is certainly not an infallible guide to phylogenetic history." (Evolutionary Biology, Douglas J. Futuyma, Third Edition, 1998 ISBN: 0-87893-189-9, p 651-653) |
|
|
|
|
BIOLOGY
Helena Curtis
1968 |
"Embryology and Evolution: Not long after the establishment of evolutionary theory, the great German biologist E. H. Haeckel, on the basis of his studies of the embryos of a number of organisms, proposed that each organism as it grows from the one-celled egg to the multicelled individual passes through all the evolutionary stages that preceded it-that is, "ontogeny (development) re-capitulates phylogeny," or, to put it more simply, "each animal climbs up its family tree." According to this theory, the mouse embryo might first resemble a one-celled amoeba, then a one-layered Volvox, then a two-layered coelenterate, and so on, right through fish and amphibian. This intriguing idea gained widespread popularity since much evidence, such as the gill slits of mammals, seemed to provide strong support. Modern biologists realize that Haeckel was only partly right. An early embryo does not resemble any previous, ancestral adult form. It does, however, bear some important resemblances to the embryos of its evolutionary predecessors, and it is to these predecessors of the mouse that we shall now turn for clues, We shall begin with the amphioxus, where chordate history traditionally commences." (BIOLOGY, Helena Curtis, 1968, ISBN 68-18187, p516) |
|
|
|
|
Fundamental concepts of Biology, Nelson Robinson, Boolootian, 1970, p279, isbn: 75-100329 |
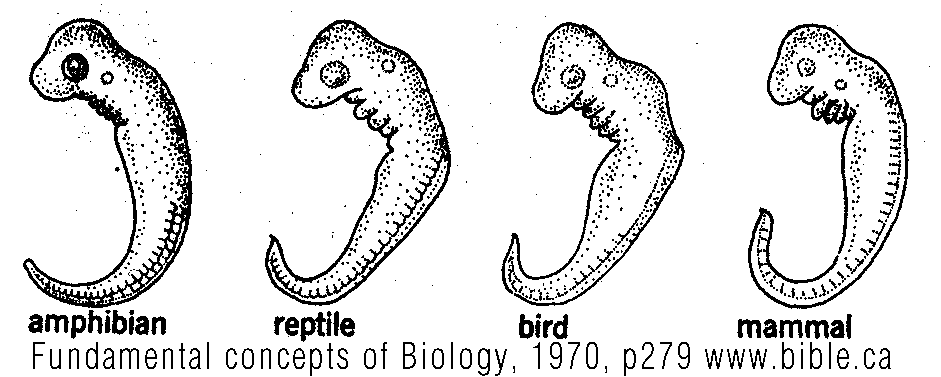
"Figure 20.7. All vertebrates have similar gill slits as embryos (upper diagrams). The different fates of the structures associated with the gill slits in a shark and in man are illustrated in the lower diagrams." ... "This basic limb plan is not clearly evident in a highly specialized animal like a horse until we ex-amine the animal's early developmental stages. At this time many structural features appear in generalized form before the animal develops the special adaptations that characterize it as a horse. For this reason, embryology has been one of the most useful means of unraveling confusing relationships among some animals. Structures that exhibit a similar basic form and have a common origin in the embryo are termed homologies. The vertebrate forelimbs listed earlier are all homologous structures. If we examine the basic limb plan of insects, we find no resemblance, anatomically or embryologically, to the vertebrate limb. The term analogous is applied to structures that are used for similar functions but that are not basically alike and have not arisen from the same embryonic source. Biologists interpret analogous structures to signify an absence of close kinship between two groups. This interpretation is reinforced when other anatomical systems and develop-mental patterns are compared in the two groups. Some seemingly non-adaptive structures occur in the embryos of higher organisms, but through modification become distinct adaptive structures in the adults of different groups. The gill slits of vertebrate embryos are a good example (Figure 20.7). In fish, most of the gill-slit structure develops into gills and gill slits in the adults. The embryos of amphibia, reptiles, birds, and mammals also have gill slits, but these do not develop into gills (except in some of the amphibians). In later development, parts of these structures become modified into parts of the ear and parathyroid and thymus glands. Not only does this evidence indicate a close evolutionary relationship among the many vertebrate groups, but it also seems nearly inexplicable on any other grounds. Relationships among plants are also studied by examining their structure and development. (Fundamental concepts of Biology, Nelson Robinson, Boolootian, 1970, p279, isbn: 75-100329) |
|
|
|
|
Biology, James M. Barrett, 1986 edition, Prentice-Hall, p 754 |
"FIG. 27-4 Embryonic stages of diverse vertebrates. Note similarity in the external features of early embryos of all vertebrates."

(Click on photo for high resolution)
"Embryonic Features As Evidence Of Evolutionary Relationships: The early development of an organism often reveals something of its evolutionary past. For example, vertebrates exhibit several progressive changes that can be organized into a graded series starting with the most primitive jawless fishes through bony fishes, amphibians, reptiles, birds, and mammals. The more primitive of these groups are judged to be ancestral to the more advanced ones. This view is held despite the obvious differences between fishes, which are aquatic and have gills and fins, and adult reptiles, birds, and mammals, which are air-breathing tetrapods, lack gills, and are usually highly adapted for terrestrial life. But when we compare the embryonic stages of fishes with those of the higher vertebrates, a fundamental relationship between the fishes and the higher vertebrates becomes obvious (Fig. 27-4). Embryos of all major groups of vertebrates, from fishes to mammals, possess gill pouches and gill furrows in addition to their common gross morphology. But only in fishes do these develop into functional gills. Why would the embryos of reptiles, birds, and mammals possess gill pouches and furrows even if they do not develop into gills? Ernst Haeckel, a nineteenth century German biologist, suggested that the embryonic stages of advanced organisms briefly recapitulate some of the morphological characters of their ancestors. Thus, the reason that a bird embryo bears gill pouches and furrows is because a group of ancient fishes were ancestral to birds. As Haeckel put it, "ontogeny [development] recapitulates phylogeny [evolution]." This does not, however, imply that all embryonic features can be interpreted as recapitulatory. For example, embryos of reptiles, birds, and mammals develop the embryonic membranes amnion, chorion, and allantois. Rather than representing equivalent structures possessed by ancestors, these are newly evolved structures important for survival of embryos on land. (Biology, James M. Barrett, 1986, ISBN 0-13-076597-X, Prentice-Hall, p 754) |
|
|
|
|
BIOLOGY - The Living Science by Miller and Levine (Lion)
BIOLOGY (Elephant)
1999 |
"Haeckel's Fraud Rediscovered! In the 19th Century, German Biologist Ernst Haeckel developed a concept known as "Ontogeny Recapitulates Phylogeny." In brief, this means that an organism's embryonic development gives us a instant replay of its evolutionary history. Biologists have known for some time that this idea was clearly not correct, but it turns out that the drawings of embryos Haeckel used to support his argument were not accurate. To put it bluntly, they were frauds. (BIOLOGY by Miller & Levine). This idea has been pushed back into the news recently by the news that Haeckel's drawings of embryonic similarities were not correct. British embryologist Michael Richardson and his colleages published an important paper in the August 1997 issue of Anatomy & Embryology showing that Haeckel had fudged his drawings to make the early stages of embryos appear more alike than they actually are! As it turns out, Haeckel's contemporaries had spotted the fraud during his lifetime, and got him to admit it. However, his drawings nonetheless became the source material for diagrams of comparative embryology in nearly every biology textbook, including ours! Joe Levine is a biologist, educator, and science journalist. He is the author of six books and numerous articles on scientific subjects, and the co-author (with Kenneth Miller) of two widely acclaimed biology textbooks for high school and college students. Page 223 of the Lion Book (BIOLOGY - The Living Science) and page 283 of the Elephant Book (BIOLOGY by Miller and Levine) each contain drawings of the early stages of embryonic development in several vertebrates. Although neither of these drawings are identical to his, they are based on the work of Ernst Haeckel, a 19th century German Biologist who was a pioneer in the study of embryonic development. Joe Levine and I (Ken Miller) are now in the process of revising the drawings that appear on these pages of our textbook, and we hope to have our publisher correct them in the very next printing of the book." by Kenneth Miller, Author (continued next section:) |
|
|
|
|

Biology, Miller and Levine Prentice Hall, 2000 Edition, p283) |
"So, what have we done? Well, we fixed it. Joe Levine and I have now revised the drawings that appear on these pages of our textbooks, and the 5th Edition of the Elephant book has been published with an accurate drawing of the embryos made from detailed photomicrographs. We have also rewritten page 283 of the 5th edition to better reflect the scientific evidence regarding the similarities of early development:" by Kenneth Miller, Author
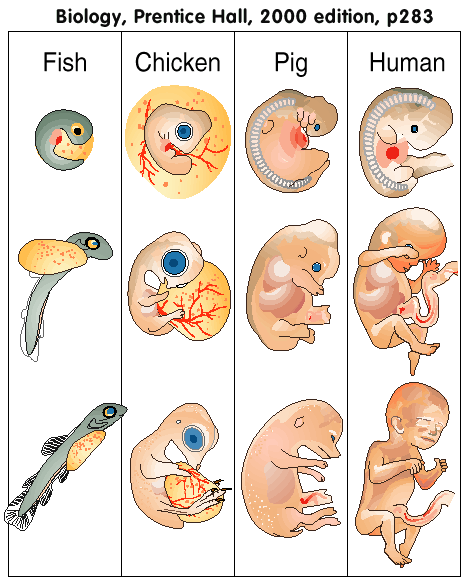
"13-4 Evidence from Living Organisms: Fossils of extinct organisms are not the only evidence that shows the ongoing process of evolution. All living organisms bear traces of the history that links them to their ancestors.
Similarities in Early Development : In the late nineteenth century, scientists noticed that the embryos of many different animals looked so similar that they were hard to tell apart. Embryos are organisms at early stages of development. This does not mean that a human embryo is identical to a fish or a bird embryo. However, as you can see in Figure 13-16, many embryos are similar in appearance, especially during the early stages of development. What do these similarities mean? The similarities of vertebrate embryos show that similar genes are at work. The genes that control an animal's basic body plan-its head and tail, its right and left, and even the positions of its limbs-are strikingly similar. In fact, a particular group of genes, known as the Hox cluster, establishes the basic pattern of organs and structures arranged from an animal's head to its tail. The common patterns of embryonic development we see in vertebrates occur because all these animals share the same basic control mechanism. If this principle is true, [note: this is not proven, but theory, ie wishful thinking] then what accounts for the differences between species? This is where evolution comes in. The common ancestors of these animals passed on a single genetic pattern of development to their descendants. Evolution, however, acted on mutations, which are changes in the genetic blueprint in an organism's DNA. Over time, these changes produced animals whose adult bodies were as different from each other as fishes and horses are. However, any mutation that produced a large disruption in the pattern of development was probably lethal and was eliminated by natural selection. In contrast, successful mutations-ones that produced favor-able structural changes in the adult without breaking down the pattern-were likely retained. As a consequence, genes that control the earliest stages of development in general remain relatively unchanged. Thus the embryos of different species resemble each other. The similarities and differences in embryonic development, which reflect the ancestry of each group of animals, provide additional evidence for evolution. Similarities in Body Structure: In the embryos of many animals-humans, birds, horses, and whales, for example-the clumps of cells that develop into limbs look quite similar. But as the embryos mature, the limbs grow into arms, wings, legs, and flippers that differ greatly in form and function. Guide For Reading 1. What is the best explanation for the structural and biochemical similarities that exist in living organisms? 2. How do similarities in embryo development support the concept of common descent? 3. What is a homologous structure? 4. How does biochemistry provide evidence for evolution? Figure 13-16 During certain embryological stages, vastly different organisms show similarities. During later stages of development, however, profound changes occur. Thus the adults bear little resemblance to one another." (Biology, Miller and Levine Prentice Hall, 2000 Edition, p283) |
|
|
|
|
"The Way Life Works"
Hoagland & Dodson
1995 |
This reference work still used Haeckel's drawings but took the trouble to colour them! (The Way Life Works, Hoagland & Dodson, 1995) |
|
|
|
|
Additional textbooks that use Haekel's drawings not featured on this website.
(There are hundreds) |
- Evolution, Edward O. Dodson, 1960, p 46-47
- General Biology, William Bloom and Carl Krekeler, 1962 p 442
- General Zoology, Tracy Storer and Robert Usinger, 1965, p 244
- Elements of Zoology, Tracy Storer, Robert Usinger, and James Nybakken, 1968, p 216
- General Zoology, Claude Ville, Warren Walker, Jr., and Frederick Smith, 1968, p. 677
- Illustrated Origin, Richard Leakey, 1971
|
|
|
|




